Name: Boron Symbol: B Atomic Number: 5 Atomic Mass: 10.811 amu Number of Protons/Electrons: 5 Number of Neutrons: 6 Date of Discovery: 1808 Discoverer: Sir Humphry Davy, J.L Gay-Lussac.
Molar mass of BF3 = 67.8062096 g/mol
Convert grams Boron Trifluoride to moles or moles Boron Trifluoride to grams
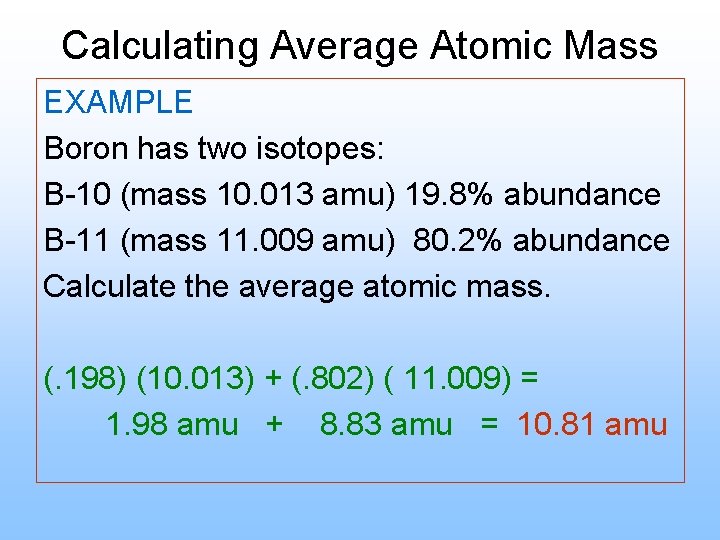
Boron Atomic Mass Formula
Molecular weight calculation:
10.811 + 18.9984032*3
- Phil Riddel Date: February 11, 2021 The Periodic Table of Elements. The atomic structure of boron, element number 5 in the periodic table, displays a full inner shell of two electrons, with three electrons in the outermost shell, giving the atom three valence electrons available for bonding.
- It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope B-17 (Boron, atomic number Z = 5, mass number A = 17).
| Symbol | # of Atoms | Boron | B | 10.811 | 1 | 15.944% | |
| Fluorine | F | 18.9984032 | 3 | 84.056% |
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Boron Atomic Mass 11
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.


Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Chemical properties of boron - Health effects of boron - Environmental effects of boron
|
BoronBoron is a non metallic element and the only non-metal of the group 13 of the periodic table the elements. Boron is electron-deficient, possessing a vacant p-orbital. It has several forms, the most common of which is amorphous boron, a dark powder, unreactive to oxygen, water, acids and alkalis. It reacts with metals to form borides. Applications The most economically important compound of boron is sodium tetraborate decahydrate Na2B4O7 · 10H2O, or borax, used for insulating fiberglass and sodium perborate bleach. Boric acid is an important compound used in textile products. Boron in the environment Boron is not present in nature in elemental form. It is found combined in borax, boric acid, kernite, ulexite, colemanite and borates. Vulcanic spring waters sometime contains boric acids. Health effects of boron
Environmental effects of boron
Now visit our boron in water page Back to chart periodic elements |
More from 'Elements'
Lenntech (European Head Office)

Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
What Is Boron Atomic Mass
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
